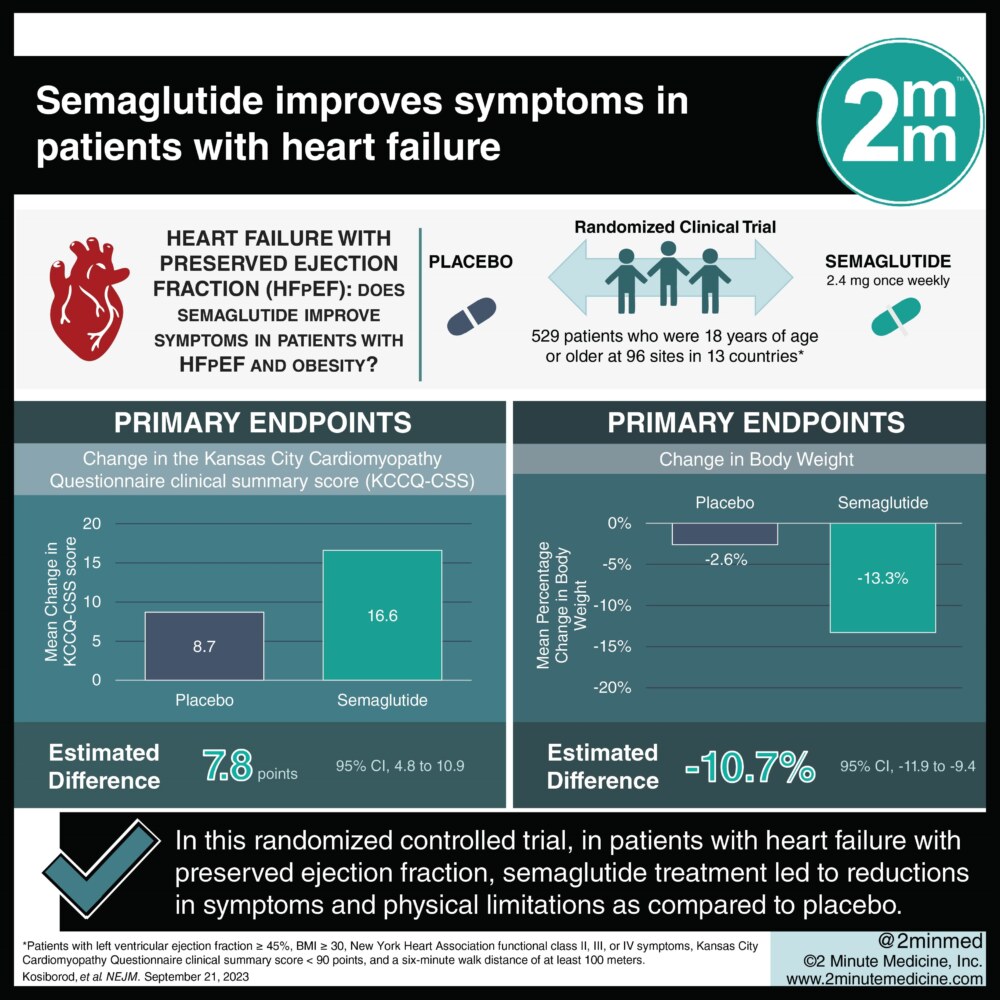
1. In this randomized controlled trial, in patients with heart failure with preserved ejection fraction semaglutide treatment led to reductions in symptoms and physical limitations as compared to a placebo.
2. In patients with heart failure with preserved ejection fraction, semaglutide treatment led to greater improvements in exercise function and weight loss as compared to a placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Patients who have heart failure with preserved ejection fraction often have multiple comorbidities such as obesity and hypertension, which leads to severely impaired quality of life and adverse hemodynamic and clinical features. Semaglutide has been shown to be effective in weight loss in persons who have an elevated body mass index (BMI). However, there is a gap in knowledge as to understanding whether once weekly semaglutide would lead to reductions in symptoms and physical limitations and to improved exercise function, in addition to weight loss, in patients with heart failure with preserved ejection fraction and obesity. Overall, this study found that in patients with heart failure with preserved ejection fraction and obesity, treatment with once-weekly semaglutide led to larger reductions in heart failure-related symptoms and physical limitations, greater improvements in exercise function, and greater weight loss than placebo. This study was limited by having a low number of non-White participants which can limit generalizability, as well as not being adequately powered to evaluate hospitalizations and urgent visits. Nevertheless, these study’s findings are significant, as they demonstrate that once-weekly semaglutide for patients with heart failure with preserved ejection fraction can lead to significant improvements in exercise and weight loss as well as reductions in heart failure-related symptoms.
Click to read the study in NEJM
Relevant Reading: Heart Failure with Preserved Ejection Fraction — A Metabolic Disease?
In-Depth [randomized controlled trial]: This randomized, double-blind, placebo-controlled trial was conducted at 96 sites in 13 countries. Patients who were 18 years of age or older with a left ventricular ejection fraction of at least 45%; a BMI of at least 30; New York Heart Association functional class II, III, or IV symptoms; a Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS) of less than 90 points, and a six-minute walk distance of at least 100 meters. Patients who had a patient-reported change in body weight of more than 5kg within 90 days before screening and a history of diabetes were excluded from the study. The primary outcomes measured were the change in the KCCQ-CSS and the percentage change in body weight from baseline to week 52. Outcomes in the primary analysis were assessed via the intention to treat with the principle of analysis of covariance. Based on the primary analysis, mean change in the KCCQ-CSS was 16.6 points with semaglutide and 8.7 points with placebo (estimated difference, 7.8 points; 95% confidence interval [CI], 4.8 to 10.9), and the mean percentage change in body weight was −13.3% with semaglutide and −2.6% with placebo (estimated difference, −10.7 percentage points; 95% CI, −11.9 to −9.4). In summary, this study demonstrates that once-weekly semaglutide treatment in patients with heart failure with preserved ejection fraction can lead to reductions in symptoms and physical limitations as well as greater weight loss as compared to placebo.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.