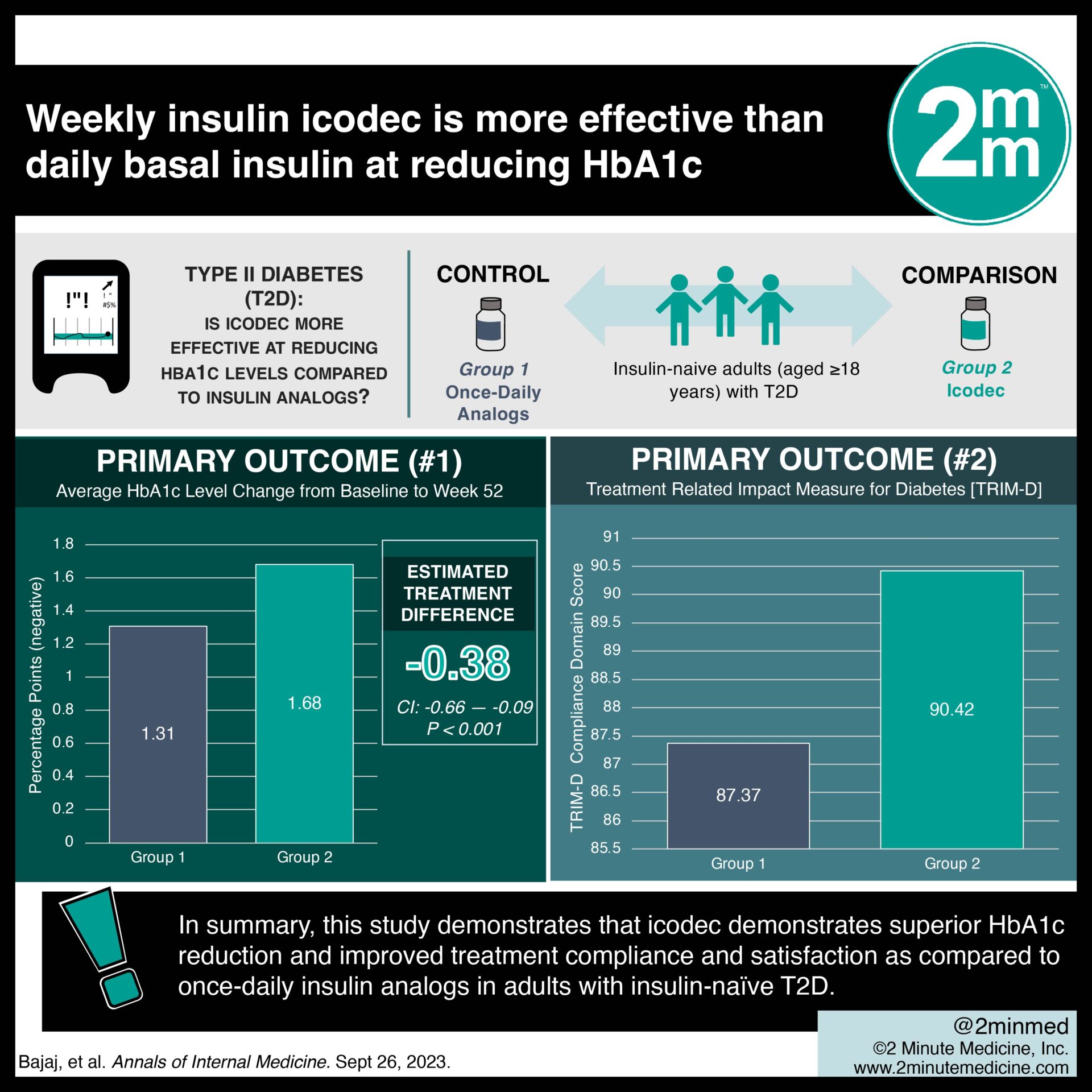
1. In this randomized controlled trial, insulin icodec demonstrated superior HbA1c reduction as compared to once-daily basal insulin analogs in insulin-naïve type two diabetes (T2D).
2. Insulin icodec showed improved treatment satisfaction and compliance as compared to once-daily basal insulin analogs in insulin naïve T2D.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Two prior phase three randomized controlled trials showed the efficacy and safety of once-weekly insulin icodec as compared to once-daily insulin glargine in adults with insulin-naïve T2D. However, there is a gap in knowledge as to understanding the effects of real-world elements to evaluate long-term glycemic effectiveness, as that can improve generalizability and provide a robust evaluation of effectiveness and safety. Overall, this study found that over one year in insulin-naïve persons with T2D, once-weekly icodec showed superiority to once-daily insulin analogs in reducing HbA1c levels with improved treatment satisfaction and compliance scores. This study was limited by being an open-label trial design as well as only being 52 weeks long, which is insufficient to assess true long-term diabetes-related and cardiovascular outcomes related to the use of icodec. Nevertheless, these study’s findings are significant, as they demonstrate that once weekly icodec can be effective in reducing HbA1c levels and improving compliance in adults with insulin naïve T2D.
Click to read the study in AIM
In-Depth [randomized controlled trial]: This 52-week, randomized, open-label, multinational, phase 3a randomized clinical trial was conducted across 176 sites in seven countries. Patients who were insulin-naive adults (aged ≥18 years) with T2D who required insulin initiation, had a glycated hemoglobin level above 7.0% (>53.0 mmol/mol), and were taking any noninsulin glucose-lowering medication were eligible for the study. Patients who had a medical condition that jeopardized their safety were excluded from the study. The primary outcome measured was the change in HbA1c level from baseline to week 52. Outcomes in the primary analysis were assessed via analysis of covariance. Based on the primary analysis, the average HbA1c level that changed from baseline to week 52 was greater with icodec as compared with the once-daily analogs (estimated treatment difference (ETD), -0.38 percentage points; 95% Confidence Interval, -0.66 to -0.09 percentage points). Additionally, after one year of being in the trial, using icodec resulted in more favorable patient-reported outcomes than with once-daily analogs. In summary, this study demonstrates that icodec demonstrates superior HbA1c reduction and improved treatment compliance and satisfaction as compared to once-daily insulin analogs in adults with insulin-naïve T2D.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.