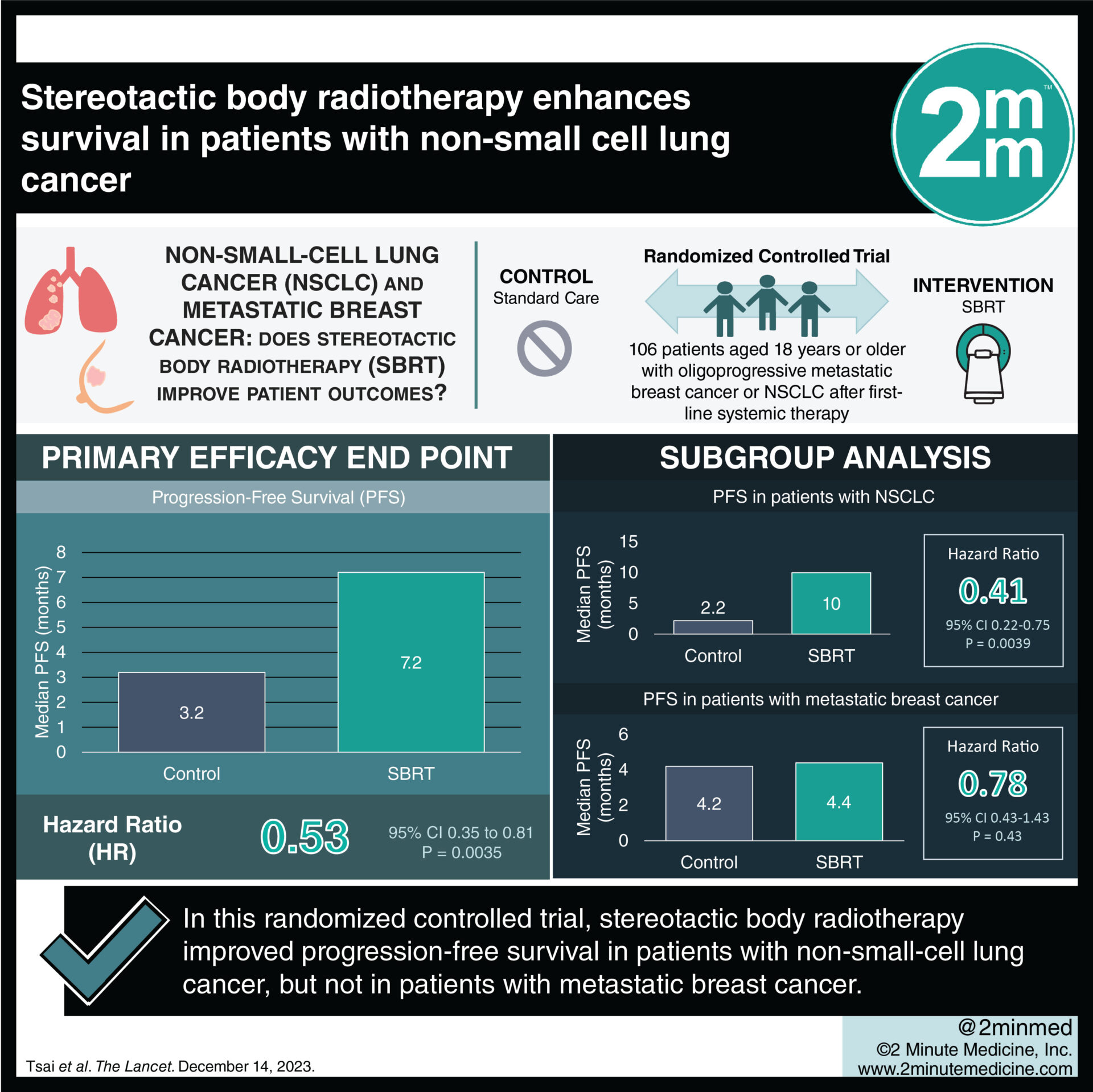
1. Median progression-free survival was greater in the SBRT group than in the standard-of-care group.
2. Patients with NSCLC displayed a significant survival benefit with SBRT in comparison to patients with breast cancer.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Most patients with metastatic breast cancer develop resistance to systemic therapy. Stereotactic body radiotherapy (SBRT) is a relatively novel technique that may limit disease progression, although its evidence in non-small-cell lung cancer and metastatic breast cancer patients has been limited. This randomized controlled trial aimed to evaluate progression-free survival (PFS) in patients with oligoprogressive metastatic breast cancer or non-small-cell lung cancer (NSCLC) receiving SBRT plus standard care versus standard care alone. The primary outcome was progression-free survival at 12 months, while key secondary outcomes were overall survival by cohort and disease group. According to study results, the NSCLC group showed significant survival benefits with SBRT compared to standard care. There was no difference in survival for patients with metastatic breast cancer who received SBRT plus standard of care or standard of care only. Although this study was well done, it was limited by the closure of the trial prior to achieving the target sample size, potentially impacting robustness.
Click to read the study in The Lancet
Relevant Reading: Breast-Conserving Surgery with or without Irradiation in Early Breast Cancer
In-depth [randomized-controlled trial]: Between Jan 1, 2019, and Jul 31, 2021, 107 patients were screened for eligibility across 7 hospital and regional centers in the USA. Included were patients ≥ 18 or older with oligoprogressive metastatic breast cancer or NSCLC after first-line systemic therapy. Altogether, 106 patients (51 to standard-of-care, 55 to SBRT plus standard-of-care) were included in the final analysis. The primary outcome showed significantly increased median progression-free survival in the SBRT group (7.2 months) compared to standard of care (3.2 months, hazard rate [HR] 0.53, p=0.0035). Notably, NSCLC patients in the SBRT group exhibited a substantial improvement (10.0 months vs. 2.2 months, HR 0.41, p=0.0039), while no benefit was observed in breast cancer patients (4.4 months vs. 4.2 months, p=0.43). Findings from this study suggest that SBRT plus standard-of-care significantly enhances progression-free survival in oligoprogressive NSCLC but does not provide similar benefits for breast cancer.
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.