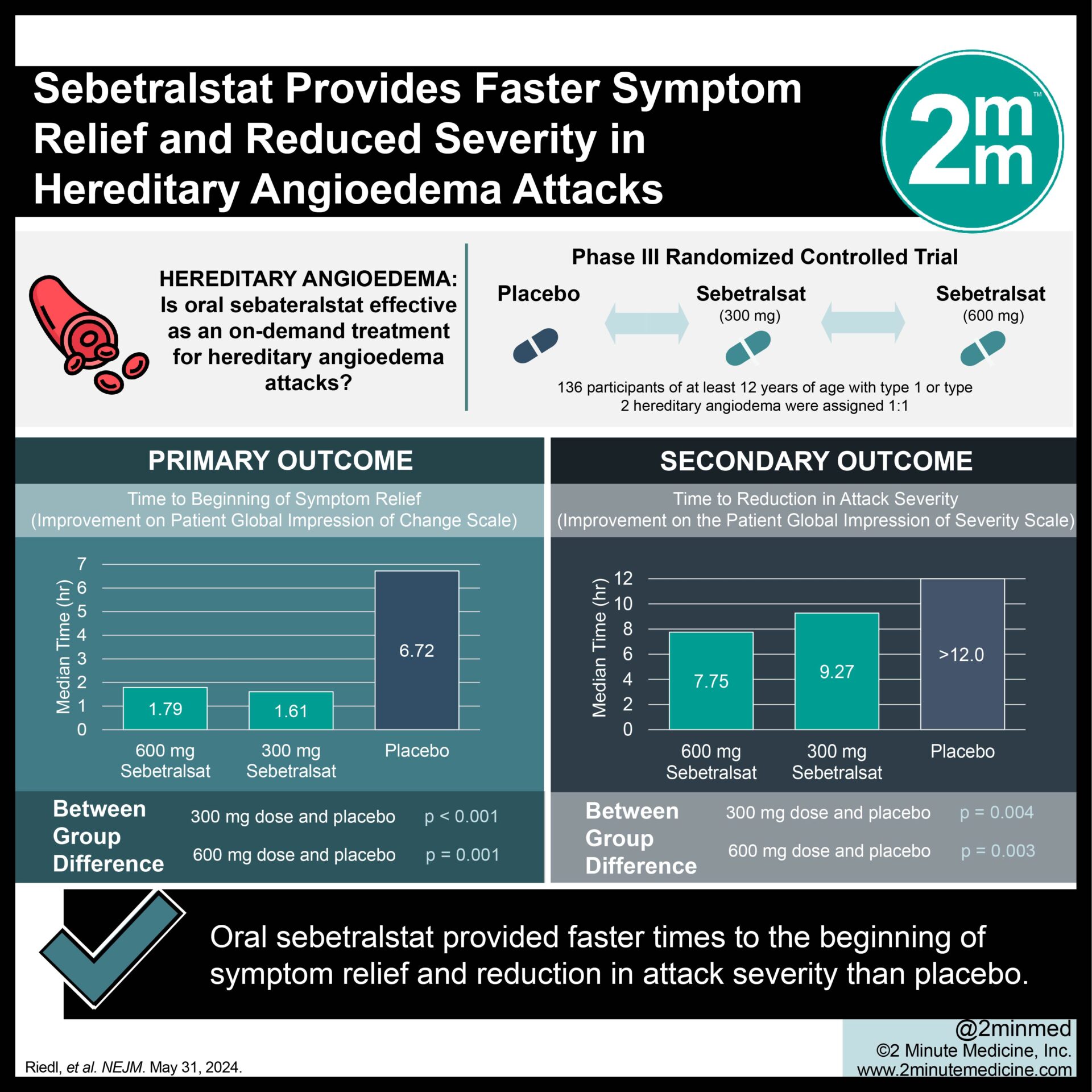
1. In this randomized controlled trial, oral sebetralstat provided faster times to the beginning of symptom relief than placebo in individuals experiencing hereditary angioedema attacks.
2. Oral sebetralstat also provided larger reductions in attack severity and faster times to complete attack resolution.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Hereditary angioedema is an autosomal dominant genetic disorder that can lead to unpredictable and debilitating episodes of tissue swelling, especially if manifested by laryngeal edema attacks. It usually presents during an individual’s adolescence and is most often caused by mutations in SERPING1, leading to a C1-esterase inhibitor deficiency (type 1) or inhibitor dysfunction (type 2). Global treatment guidelines for hereditary angioedema recommend that patients treat all attacks as early as possible, regardless of location. Current first-line options for acute treatment all require parenteral administration, leading to issues of adherence to and delays in on-demand treatment. While oral therapies have been approved for prophylaxis, none have been approved for acute treatment of angioedema attacks. Sebetralstat is an oral plasma kallikrein inhibitor that ultimately inhibits the release of bradykinin, the vasodilator responsible for the aggressive tissue swelling seen in these attacks. In a phase 2 trial, sebetralstat was associated with fast relief of symptoms and an encouraging side-effect profile. This phase 3 trial aimed to assess the efficacy and safety of sebetralstat for the acute treatment of angioedema attacks in individuals with hereditary angioedema. Overall, it showed that oral sebetralstat provided faster times to the beginning of symptom relief than placebo in individuals experiencing an attack. Sebetralstat also provided larger reductions in attack severity and faster times to complete attack resolution than placebo. The trial was limited by a relatively small sample size, attributable to the condition’s rarity.
Click to read the study in NEJM
In-Depth [randomized controlled trial]: KONFIDENT is a double-blind, randomized-controlled, three-way crossover trial that investigated the efficacy and safety of up to 2 doses of sebetralstat for treating hereditary angioedema attacks. Eligible participants were 12 years of age or older, had a diagnosis of type 1 or type 2 hereditary angioedema, and had at least two attacks in the previous three months. Enrolled participants were randomly assigned in a 1:1:1:1:1:1 ratio to administer sebetralstat at doses of 300 mg and 600 mg and placebo to themselves in a prespecified order for three consecutive attacks and to assess their symptoms at pre-specified time intervals. The primary endpoint was the time to the beginning of symptom relief, defined as the rating of “a little better” on the Patient Global Impression of Change scale at two or more consecutive time points within 12 hours of the initial administration of treatment. A total of 136 participants were enrolled in the trial, of which 110 were administered the assigned trial agent for at least one attack. The median time to the beginning of symptom relief was 1.61 hours (interquartile range, 0.78 to 7.04; p<0.001) with the 300mg dose, 1.79 hours (interquartile range, 1.02 to 3.79; p=0.001) with the 600mg dose, and 6.72 hours (interquartile range, 1.34 to >12) with placebo. The median time to the reduction in attack severity was 9.27 hours (interquartile range, 1.53 to >12; p=0.004), 7.75 hours (interquartile range, 2.19 to >12; P=0.003), and more than 12 hours (interquartile range, 6.23 to >12) respectively. Overall, oral sebetralstat was more effective in achieving faster times to the beginning of symptom relief, reducing attack severity, and rendering faster times to complete attack resolution than placebo.
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.