2. There was a concerning trend favouring placebo when assessing overall survival, suggesting a negative impact of pazopanib.
Evidence Rating Level: 1(Excellent)
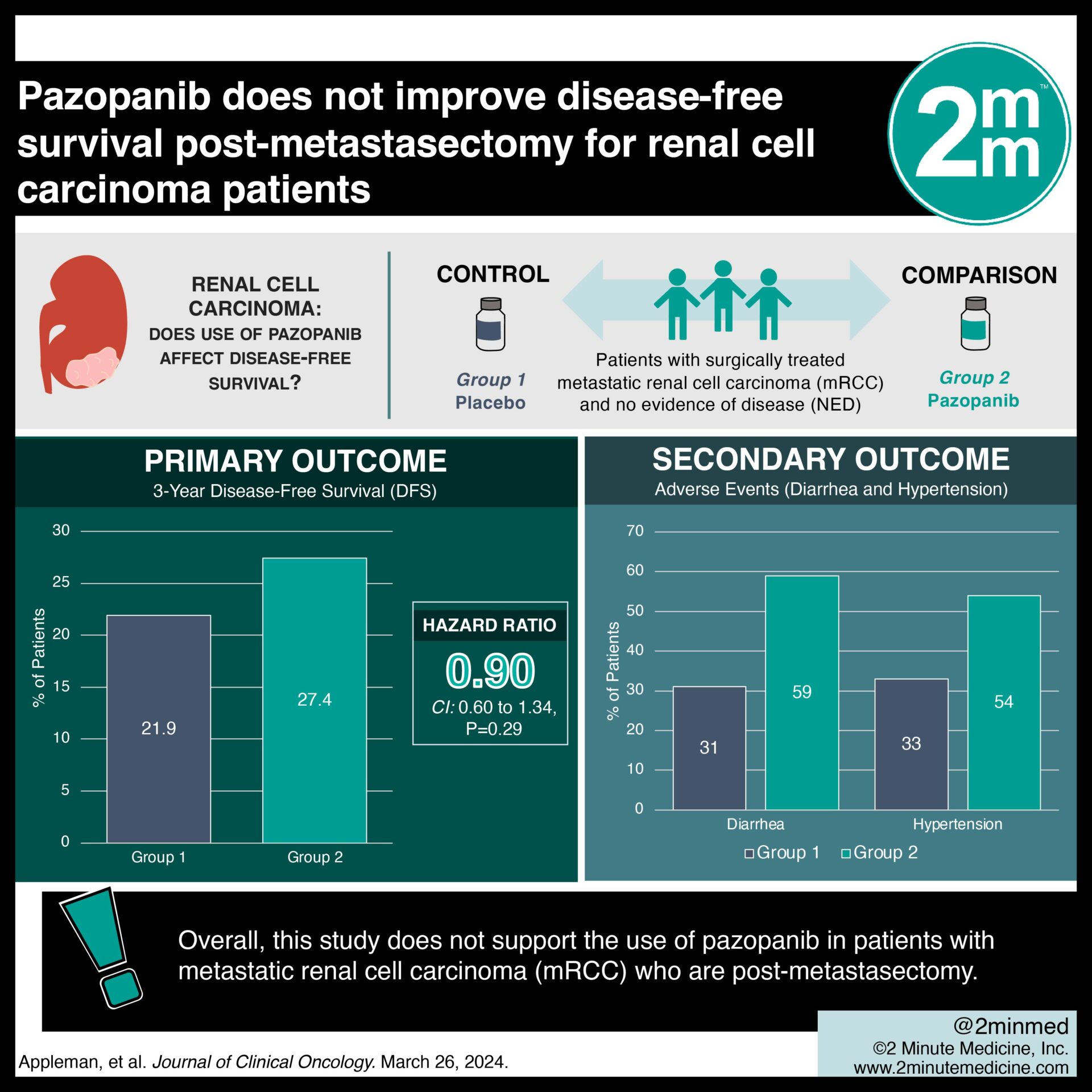
Study Rundown: The E2810 trial was a randomized, double-blind phase III trial assessing the impact of pazopanib on patients with metastatic renal cell carcinoma (mRCC) who are post-metastasectomy. The study included patients with mRCC who had undergone surgery to remove the metastases and were classified with no evidence of disease. They had prior nephrectomy or partial nephrectomy for RCC primary and received no prior systemic therapy. Patients were randomized to receive either 800 mg daily of pazopanib, or placebo. Key findings from the trial showed that pazopanib post-metastasectomy in mRCC patients did not significantly improve disease-free survival (DFS) compared to placebo. Further, there was a concerning trend toward favouring those in the placebo group over those in the pazopanib group. The study’s strengths lie in its rigorous design, including double-blinding and a multicenter approach. Limitations of the trial include its relatively small sample size of 129 patients, which may impact the study’s power to detect small effect sizes. Additionally, toxicity associated with pazopanib may have influenced the effectiveness of blinding, potentially confounding study conduct. Overall, this study does not support the use of pazopanib in patients with metastatic renal cell carcinoma (mRCC) who are post-metastasectomy.
Click to read the study in Journal of Clinical Oncology
In-Depth [randomized controlled trial]: The E2810 trial examined the efficacy of pazopanib versus placebo in patients with surgically treated metastatic renal cell carcinoma (mRCC) and no evidence of disease (NED). The trial enrolled a total of 129 patients who had not undergone prior systemic therapy. Patients were either randomized to receive daily pazopanib or placebo. The primary endpoint, disease-free survival (DFS). Overall, there was no significant difference between the pazopanib and placebo arms, with 3-year DFS rates of 27.4% and 21.9%, respectively (HR = 0.90, 95% CI 0.60 to 1.34, P = .29). Concerningly, there was a trend towards improved overall survival in patients in the placebo group (3-year OS 81.9% vs. 91.4% for placebo, HR = 2.55, P = .012). Of note, the study’s small samples size was not powered to confirm this endpoint. Adverse events were more common in the pazopanib group including diarrhea (59% vs. 31%) and hypertension (44% vs. 54% for placebo). There was treatment treatment discontinuation in 24% of pazopanib-treated patients. In summary, the findings of this study do not endorse the utilization of pazopanib in individuals with mRCC who have undergone metastasectomy.
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.















