Evidence Rating Level: 1 (Excellent)
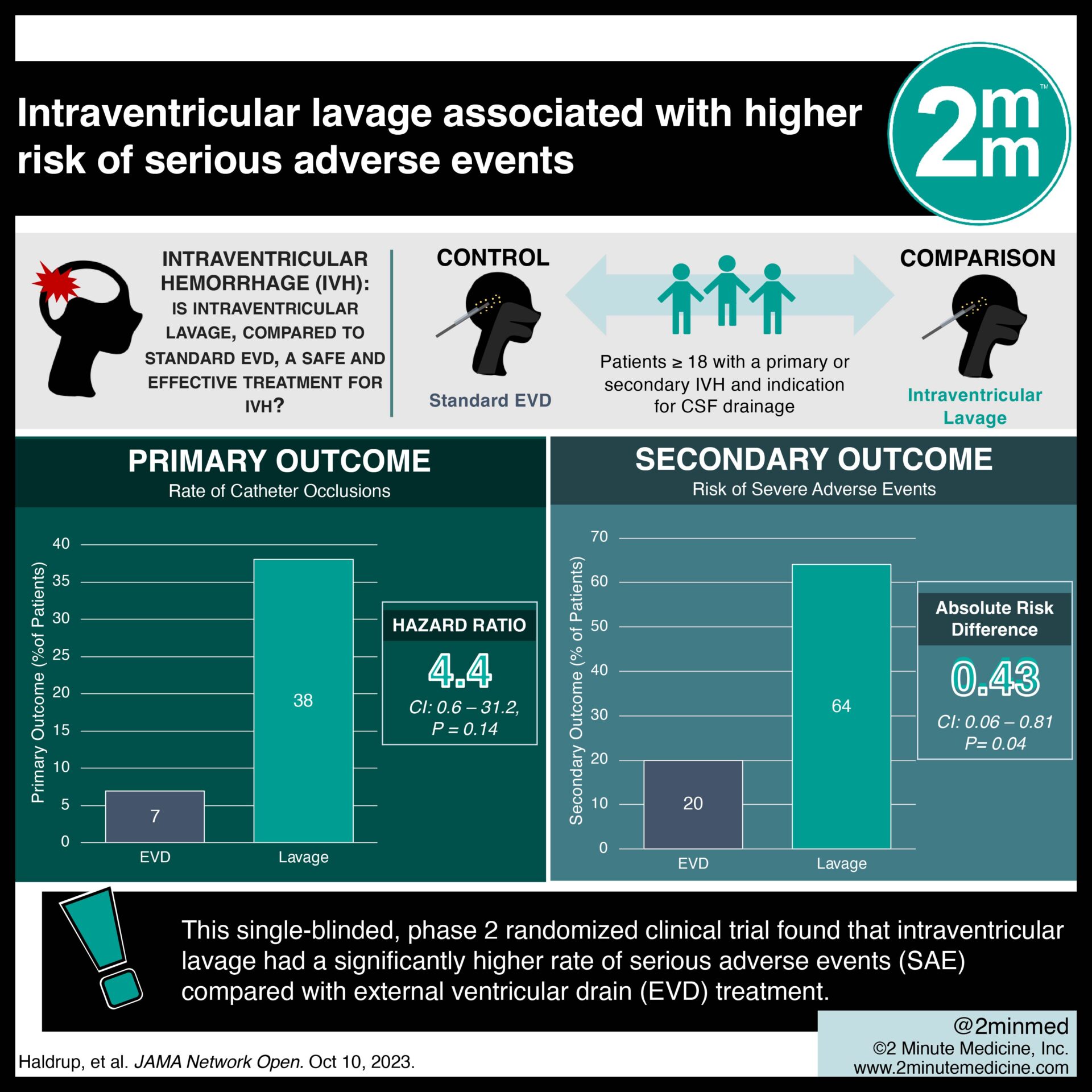
IVH—characterized by bleeding within the brain’s ventricles—is typically treated with supportive care and passive drainage using an EVD. A new intraventricular lavage technology, called IRRAflow, utilizes an active dual-lumen catheter for precise ventricular saline perfusion, aiming to speed up IVH washout and prevent complications. This single-blinded, phase 2 randomized clinical trial aims to investigate the safety and efficacy of intraventricular lavage compared with standard EVD treatment of IVH. 21 participants (median [IQR] age, 67 [59-82] years; 66% male) were randomly assigned in a 1:1 ratio to receive either intraventricular lavage (intervention) or standard EVD (control). They were blinded to their respective treatments, with 11 participants in the intervention group and 10 in the control group. The rate of catheter occlusions (primary outcome) was significantly higher in the intervention group compared with the EVD group (hazard ratio 4.4 [95% CI, 0.6-31.2]; P = .14; meeting the prespecified α = .20). The procedure time for catheter placement was significantly longer for the intervention group compared with the control group (median [IQR] time, 53.5 [33-75] minutes vs 12 [4-20] minutes; P < .001). The intervention group required significantly more time for catheter placement compared to the control group (median [IQR] time, 53.5 [33-75] minutes vs 12 [4-20] minutes; P < .001). Based on an interim analysis of the first 20 participants, the intervention group faced a significantly higher risk of experiencing at least 1SAE (absolute risk difference 0.43, 95% CI, 0.06-0.81; P = .04). Consequently, the study was terminated early. In terms of treatment efficacy, the hematoma clearance rate did not differ significantly between the intervention and control groups (P = .64). These findings suggest that intraventricular lavage technology has a higher risk of SAEs under the investigated conditions, emphasizing the importance of caution and technology adaptations to ensure patient safety.
Click to read the study in JAMA Network Open
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.









