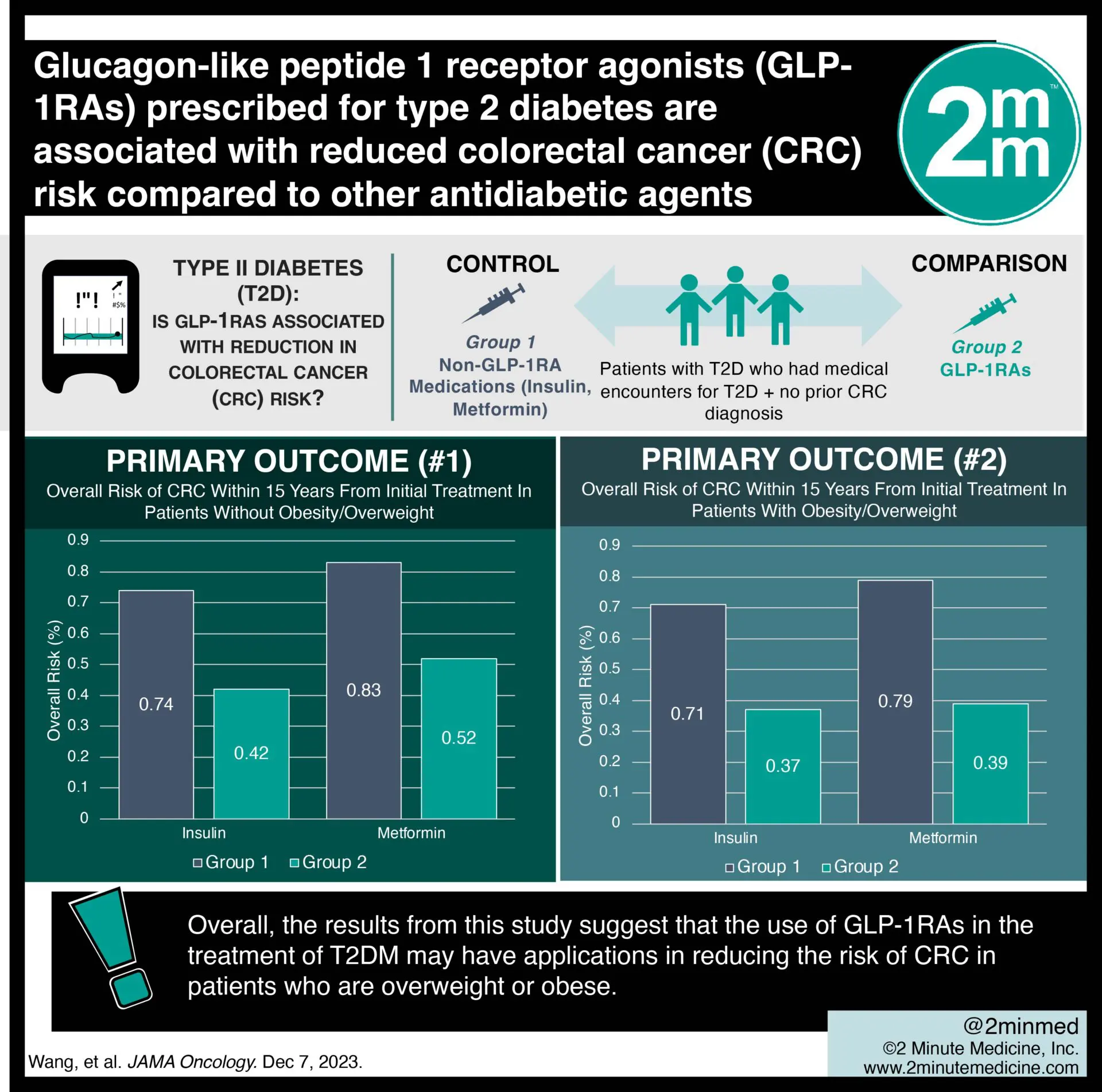
2. Results for overweight patients and those with obesity showed GLP-1RAs significantly reduced risk for CRC compared with multiple antidiabetics.
Evidence Rating Level: 2 (Good)
Study Rundown: An important risk factor for colorectal cancer (CRC) is being overweight or obese. One of the medications used to treat type 2 diabetes (T2DM) is glucagon-like peptide 1 receptor agonists (GLP-1RAs) which have multiple effects, including weight loss. This study explored whether GLP-1RA use in T2DM was associated with a reduction in CRC risk. The primary outcome of this study was the first diagnosis of CRC within 15 years from the initial treatment of GLP-1RAs or non-GLP-1RA diabetes medications (insulin, metformin, alpha-glucosidase inhibitors, dipeptidyl-peptidase-4 inhibitors (DPP-4i), sodium-glucose cotransporter-2 inhibitors (SGLT2i), sulfonylureas (SU), and thiazolidinediones (TZD)). A secondary analysis of this metric was completed after stratifying patients by overweight status. There was a significant reduction in the risk of CRC in patients who received GLP-1RAs when compared with all other antidiabetic medications, except alpha-glucosidase or DPP-4i, in which there was a non-significant reduced risk. This was consistent between the sexes. Additionally, there was a significantly lower risk of CRC in overweight and obese patients when treated with GLP-1RAs as compared with all antidiabetics examined in this study. Limitations to this study include that there were uncontrolled confounding factors, as well as bias secondary to self-selection of treatment. Additionally, it is noted that greater research is required to determine the outcomes in patients who have had previous treatment with antidiabetic medications, or whether there are different effects within the class of GLP-1RAs antidiabetic medications. Overall, the results from this study suggest that the use of GLP-1RAs in the treatment of T2DM may have applications in reducing the risk of CRC in patients who are overweight or obese.
Click to read the study in JAMA Oncology
In-Depth [retrospective cohort]: This retrospective cohort study was executed using the TriNetX network and included de-identified data from 1,221, 218 antidiabetic-naïve and CRC-negative patients who were provided medical treatment for T2DM between 2005-2019. Patients who were prescribed GLP-1RAs for treatment of their T2DM had reduced risk for CRC when compared with insulin (hazard ratio (HR), 0.56; 95% confidence interval (CI), 0.44-0.72). Similar findings were identified in comparison with metformin (HR, 0.75; 95% CI, 0.58-0.97), SGLT2i (HR, 0.77; 95% CI, 0.62-0.97), SU (HR, 0.82; 95% CI, 0.68-0.98), and TZD (HR, 0.82; 95% CI, 0.69-0.97). There was a non-significant decreased risk of CRC when compared with alpha-glucosidase or DPP-4i. In overweight patients and those with obesity provided with GLP-1RAs, there was a significant reduction in CRC risk when compared with insulin (HR, 0.50; 95% CI, 0.33-0.75), metformin (HR, 0.58; 95% CI, 0.38-0.89), SU (HR, 0.63; 95% CI, 0.48-0.82), SGLT2i (HR, 0.68; 95% CI, 0.47-0.99), TZD (HR, 0.73; 95% CI, 0.54-0.98), and DPP-4i (HR, 0.77; 95% CI, 0.59-1.00).
Image: PD
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.









