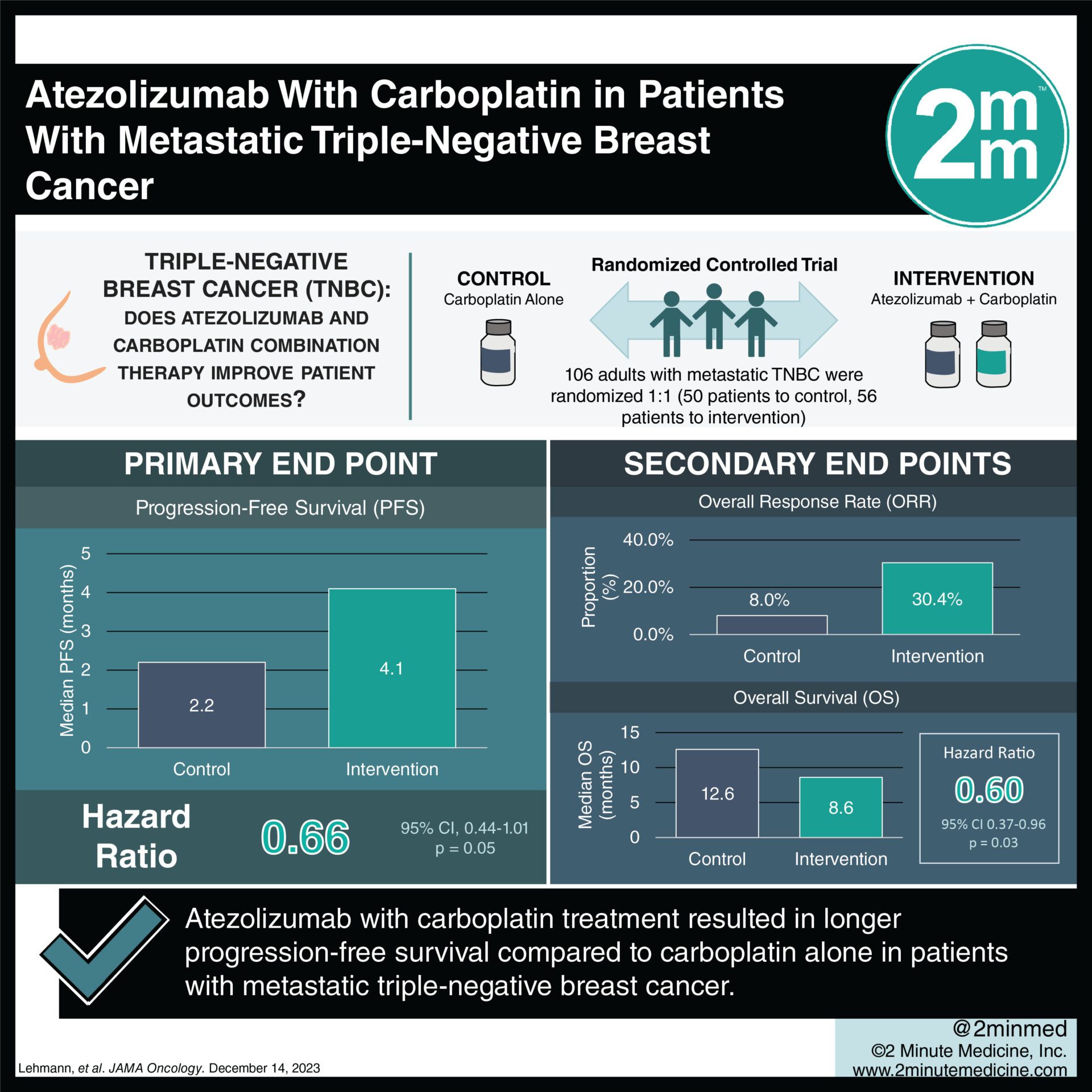
1. Progression-free survival was 4.1 months in the combination arm vs 2.2 months in the monotherapy arm with an HR of 0.66.
2. Grade 3/4 serious adverse events occurred in 41% in the combination arm vs 8% in the monotherapy arm.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Previous studies explored treating PD-L1–positive metastatic triple-negative breast cancer (TNBC) with immunotherapy (atezolizumab) and paclitaxel but found no improved outcomes. This phase II study explored treatment with atezolizumab and carboplatin, a DNA-intercalating agent, for PD-L1–positive metastatic TNBC. The primary endpoint was progression-free disease (PFS) and secondary endpoints included overall response rate (ORR), duration of response (DoR), and overall survival (OS). Median PFS was 4.1 months with atezolizumab plus carboplatin vs 2.2 months with carboplatin, with an HR of 0.66 (p=0.05). Subgroup analysis found women aged 41-64, postmenopausal women, patients with liver metastasis, patients with prior chemotherapy, patients with obesity, patients with poor glucose control, and patients with high tumor-infiltrating lymphocytes, had more significant PFS improvement. Patient outcomes did not differ significantly based on PD-L1 expression. Median OS was 12.6 months in the combination therapy compared to 8.6 months with monotherapy, with HR 0.60 (p=.03). With regards to safety, grade 3/4 serious adverse events occurred in 41% in the combination therapy vs 8% in the monotherapy, with the most common adverse events in the combination therapy including thrombocytopenia, anemia, lymphocytopenia, nausea, fatigue, and increased liver enzymes. The strengths of this study included a varied number of outcomes and measures to investigate, and the limitation included a small sample size. Overall this study found that combination therapy with atezolizumab and carboplatin had some improved outcomes when compared to carboplatin monotherapy for patients with metastatic TNBC.
Click to read the study in JAMA Oncology
Relevant Reading: Chemotherapy weakly contributes to predicted neoantigen expression in ovarian cancer
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.