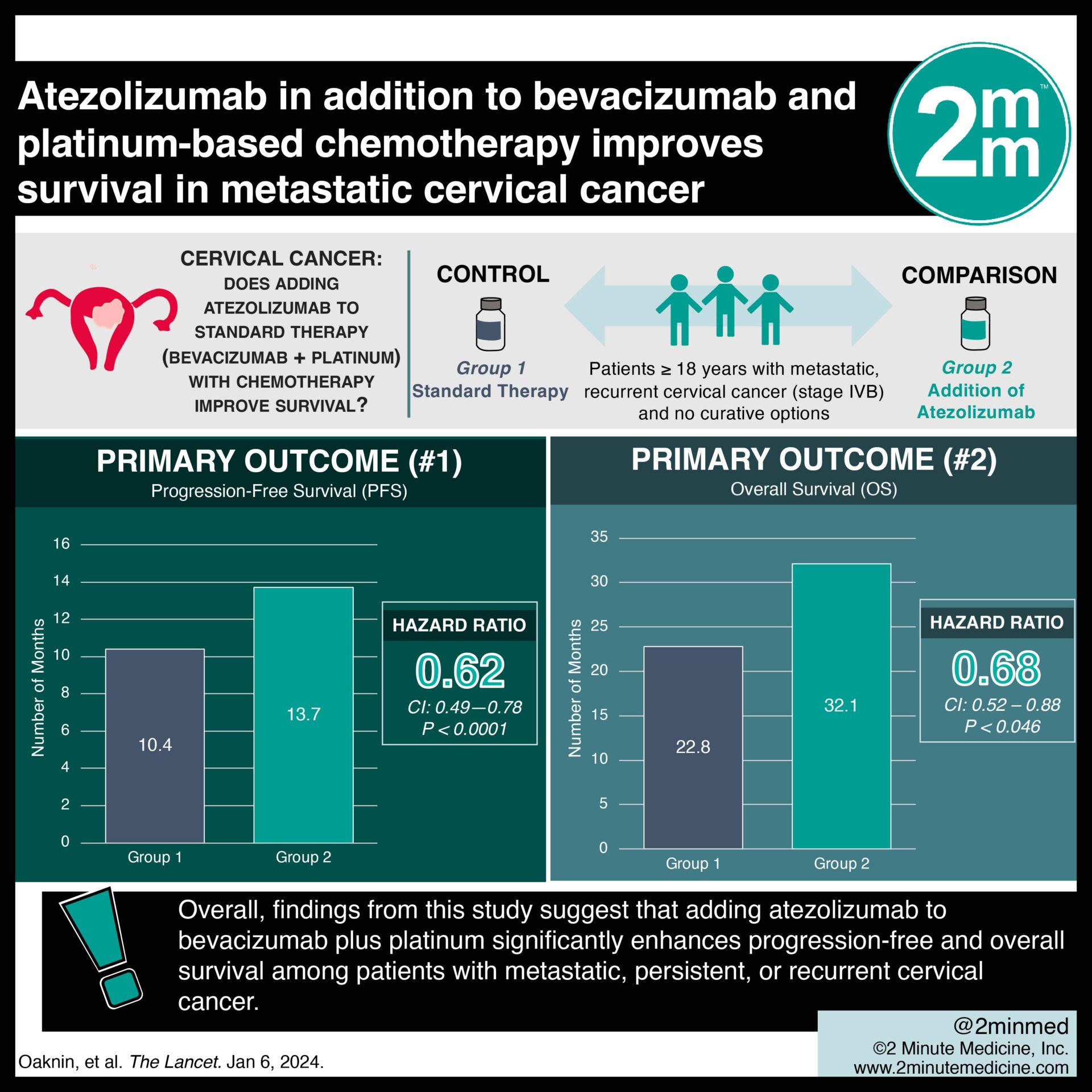
1. The Atezolizumab group demonstrated significantly greater progression-free and overall survival among patients with metastatic, persistent, or recurrent cervical cancer.
2. Grade 3 or worse adverse events were comparable in both groups.
Evidence Rating Level: 1 (Excellent)
Study Rundown: A previous large-scale trial has shown improved overall survival among patients with cervical cancer treated with bevacizumab and chemotherapy. Other studies have shown similar results using chemotherapy and PD-L1 monoclonal antibodies, such as pembrolizumab. However, the efficacy of all three therapeutic regimens combined is yet to be studied. This phase 3 randomized trial aimed to evaluate the impact of adding atezolizumab to bevacizumab plus chemotherapy on survival in patients with metastatic, recurrent cervical cancer. The primary outcome was progression-free survival, while the key secondary outcome was overall survival. According to study results, atezolizumab significantly improved both progression-free and overall survival compared to the standard regimen. This study was strengthened by a large sample size with patients from various countries, thus augmenting the external validity of the results.
Click to read the study in The Lancet
Relevant Reading: Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.