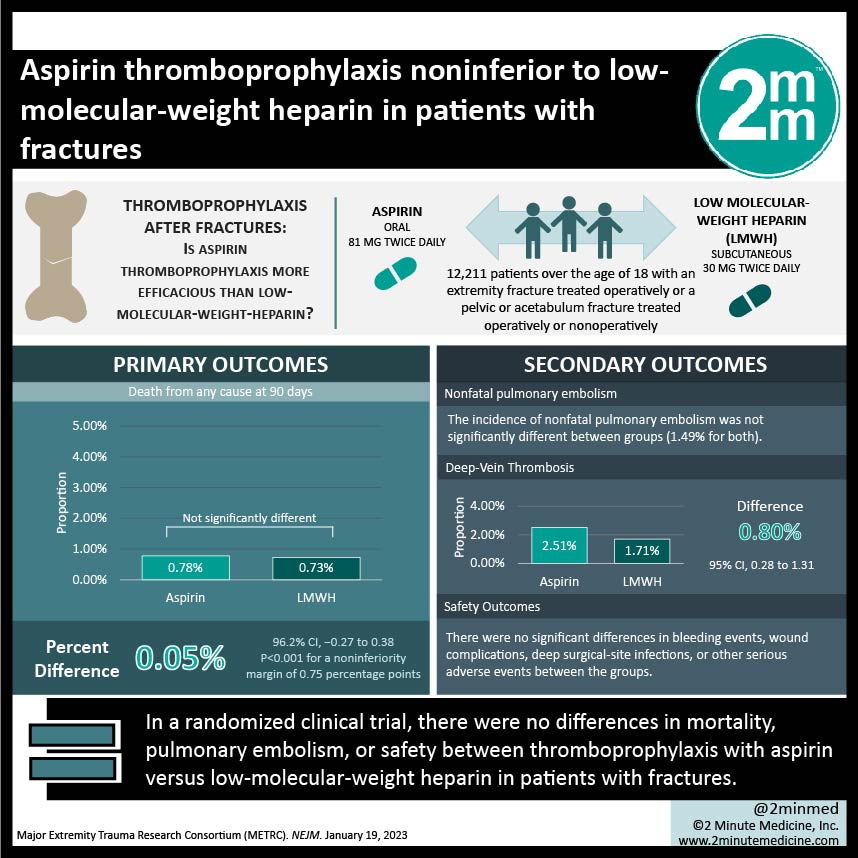
1. In this randomized clinical trial, there were no differences in mortality, pulmonary embolism, or safety between thromboprophylaxis with aspirin versus low-molecular-weight heparin in patients with fractures.
2. Incidence of deep vein thrombosis was higher with aspirin use compared to low-molecular-weight heparin use.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Thromboprophylaxis therapy is currently recommended to reduce death and complications associated with venous thromboembolism after fractures. Aspirin is often preferred over low-molecular-weight heparin by patients for thromboprophylaxis due to its lower cost and oral administration. However, there is paucity in evidence comparing aspirin and low-molecular-weight heparin treatment. In this randomized clinical trial, the efficacy and safety of aspirin and low-molecular-weight heparin for thromboprophylaxis treatment were compared in patients with an extremity, pelvic, or acetabulum fracture. Low-molecular-weight heparin was administered subcutaneously at a 30 mg dose twice daily and aspirin was given orally at an 81 mg dose twice daily. The duration of thromboprophylaxis ended or continued at discharge depending on existing clinical protocols of each hospital. Patients were followed for 90 days. Incidence of death was not significantly different between groups, even after stratifying by age or cause of death. Incidence of nonfatal pulmonary embolism was not significantly different between groups. There were significantly more deep vein thrombosis occurrences in the aspirin group compared to the low-molecular-weight heparin group. Safety outcomes, including bleeding events, wound complications, deep surgical-site infections, or other serious adverse events, were not significantly different between groups. As limitations, the study had an open-label design and there were differences in the duration of thromboprophylaxis therapy after discharge depending on hospital protocol.
Click to read the study in NEJM
In-Depth [randomized controlled trial]: This randomized controlled clinical trial compared the efficacy and safety of thromboprophylaxis treatment with aspirin and low-molecular-weight heparin in patients with fractures. Patients over the age of 18 with an extremity fracture treated operatively or a pelvic or acetabulum fracture treated operatively or nonoperatively (n = 12,211) were randomized to receive aspirin (n = 6,101) or low-molecular-weight heparin (n = 6,110). Patients were followed for 90 days. Protocol adherence was 94.7% and 96.9% for the aspirin and low-molecular-weight heparin groups, respectively. The 90-day probability of death was 0.78% and 0.73% in the aspirin and low-molecular-weight heparin groups, respectively, and not significantly different between groups. The incidence of nonfatal pulmonary embolism was not significantly different between groups. The 90-day probability of deep vein thrombosis was significantly higher in the aspirin group compared to the low-molecular-weight heparin group (2.51% and 1.71%; 0.80 percentage points difference; 95% Confidence Interval, 0.28 to 1.31). For safety outcomes, there were no significant differences in bleeding events, wound complications, deep surgical-site infections, or other serious adverse events between groups. In summary, thromboprophylaxis with aspirin was noninferior to low-molecular-weight heparin for the prevention of thromboembolic events in patients with orthopedic trauma.