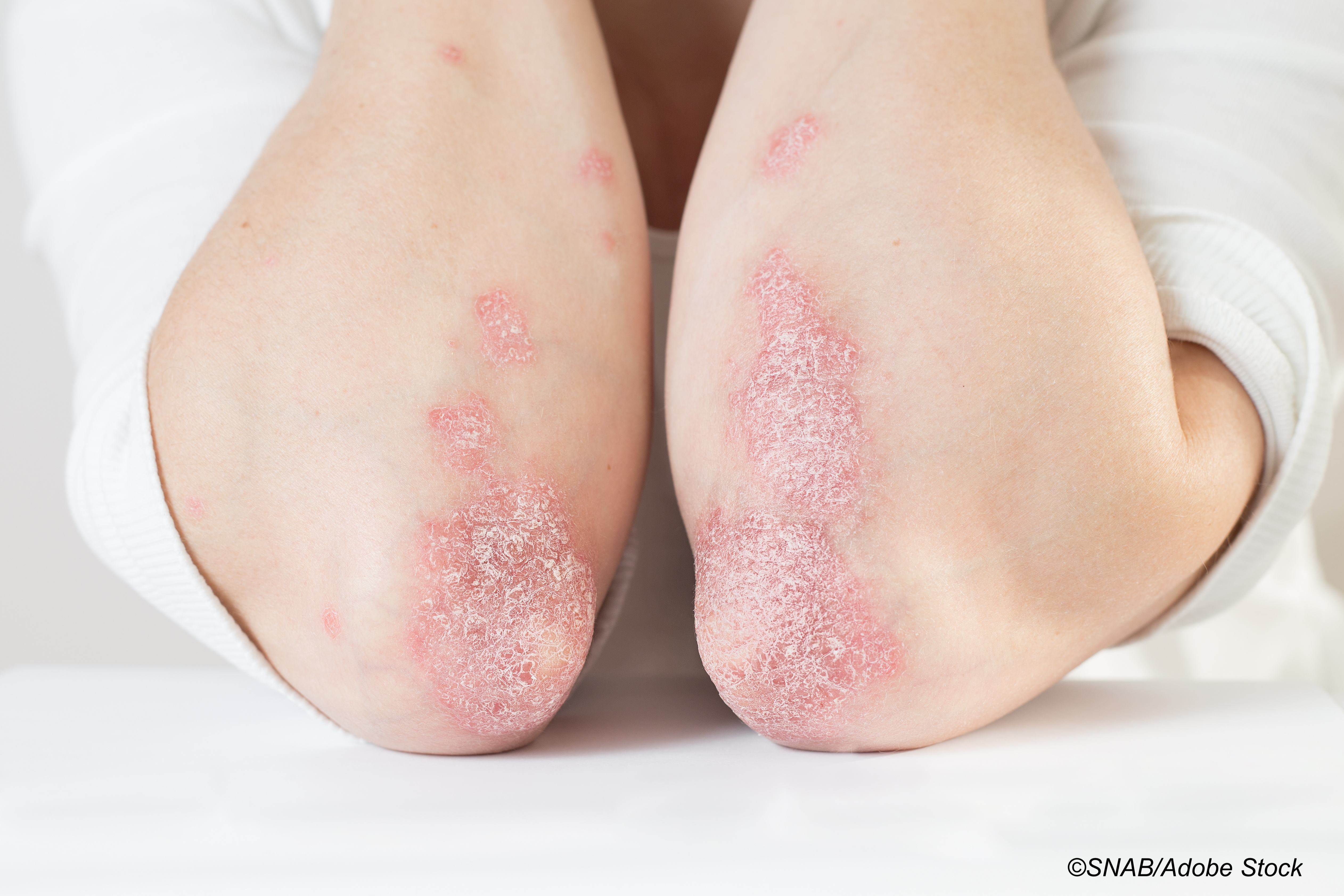
Treatment with abatacept was not effective in preventing relapse of psoriasis in patients with moderate-to-severe plaque psoriasis who had been treated with ustekinumab, according to results from the PAUSE study. Researchers concluded that psoriasis, therefore, may rely on different pathways to activate residual T-cells in the skin.
“Abatacept might not be a good choice for treating psoriatic arthritis and skin psoriasis in the same patient. It was surprising that abatacept is effective for psoriatic arthritis but did not prevent or delay a skin psoriasis relapse after stopping ustekinumb,” first author Kristina M. Harris, PhD, Biomarker and Discovery Research, Immune Tolerance Network, University of California, San Francisco, told BreakingMED, in an email correspondence.
Their findings were published in JAMA Dermatology.
“Abatacept (CTLA4-Ig) is a costimulatory-blocking fusion protein that consists of the extracellular domain of the CTLA4 ligand for CD80/CD86 coupled to a modified Fc portion of human IgG. Abatacept acts by competing for CD80/CD86 binding to CD28 on T cells, thereby inhibiting T-cell activation and function. Abatacept had a treatment effect during an early-phase psoriasis trial12 and improved skin lesions in a phase 2 psoriatic arthritis trial. Therefore, it was hypothesized that costimulatory blockade with abatacept could induce tolerance in pathogenic T cells encountering antigen in resolving psoriasis lesions, leading to long-term remission,” explained Harris and colleagues.
“Ustekinumab is highly effective for skin psoriasis, but patients nearly always relapse when ustekinumab is stopped. We wanted to find a treatment that would lead to a long-lasting remission. Abatacept targets an immune pathway known as ’costimulation’, which is different from the cytokine signaling pathway targeted by ustekinumab. The combination of targeting two immune pathways might lead to a long-lasting skin psoriasis remission. Additionally, abatacept is FDA-approved for treating psoriatic arthritis, so it might be effective in treating skin psoriasis too,” Harris told BreakingMED.
The Psoriasis Treatment with Abatacept and Ustekinumab: a Study of Efficacy (PAUSE) study was a parallel-design, double-blind, placebo-controlled randomized trial conducted at 10 sites in the U.S. and Canada, in patients with moderate-to-severe plaque psoriasis. All patients had a Psoriasis Area and Severity Index (PASI) of ≥12, and affected body surface of ≥10%.
The study was comprised of a lead-in phase (weeks 0-12), a randomized treatment phase (weeks 12-40), and an observation phase (weeks 40-88). For the lead-in phase, patients received subcutaneous ustekinumab (45 mg/dose for those ≤100 kg; 90 mg/dose for those >100 kg) at weeks 0 and 4. Patients who had a response to ustekinumab at week 12—as measured via PASI—were then randomized to either continued ustekinumab at weeks 16 and 28 and abatacept placebo weekly from weeks 12-39, or switched to abatacept (125 mg/week) from weeks 12 to 39 and ustekinumab placebo at weeks 16 and 28. Among the 108 patients (mean age: 46.1 years; 67.6% men) randomized to open-label treatment with ustekinumab, 91 were randomized to blinded treatment.
Harris et al assessed PASI every 4 weeks. For RNA sequencing, they obtained skin biopsies from active lesions and non-lesional areas at baseline, and then again at weeks 12, 24, and 40, and at the final study visit.
Between weeks 12-88, the number of patients who relapsed was greater in abatacept treatment group compared with ustekinumab (91.1% versus 87.0%, respectively; P=0.41). In addition, more patients treated with abatacept relapsed between weeks 12-40 compared with those treated with ustekinumab (55.6% versus 30.4%, respectively; P=0.01). The median time to relapse from the last dose of ustekinumab was similar between the groups (32 versus 36 weeks).
Upon RNA sequencing to assess modulation of the IL-23-mediated psoriasis molecular signature in skin by ustekinumab, Harris and colleagues found that:
“Consistent with its mechanism of action, ustekinumab improved disease-associated genes that were modulated by IL-23, including IL-17A and the pathogenic psoriasis molecular signature genes that were induced in keratinocytes by IL-17 receptor signaling. In addition, ustekinumab downmodulated a number of transcripts associated with CD28-CD80/CD86 pathway targets of abatacept therapy, including CD80, CD28, ICOS, and CTLA-4.”
In comparing week 12 with week 0 levels of specific serum cytokine levels including IL-17A, IL-22, and IL-19 (shown to be increased in both active skin lesions and blood in patients with psoriasis and associated with disease activity), they also found that ustekinumab significantly reduced these, and effected “modest” reductions in serum levels of interferon γ, IL-2, IL-10, IL-6, tumor necrosis factor, and thymic stromal lymphopoietin.
At week 12, ustekinumab also reduced psoriasis molecular signature genes in lesions that were actively resolving by two-fold, but in patients treated with abatacept, suppression of this molecular signature and IL-17A transcripts in the skin were not maintained at week 24 and/or week 40.
“[T]his result is consistent with the earlier relapse time from enrollment in the abatacept group vs the ustekinumab group,” noted Harris and colleagues.
Treatment with abatacept did not suppress the IL-23-mediated psoriasis molecular signature in lesions after the withdrawal of ustekinumab. In addition, serum IL-19 levels increased. Further, week 12 suppression of serum IL-19 levels was not maintained at week 40 in patients treated with abatacept compared with those treated with ustekinumab (27 pg/mL; 95% CI: 8-57 pg/mL; P=0.008). Serum IL-17A and IL-22 levels, however, were similar.
“[T]he results of PAUSE as reported here showed that abatacept did not prevent relapse after ustekinumab withdrawal. Most participants in both treatment groups experienced a psoriasis relapse or dropped out between weeks 12 and 88. Although abatacept is approved for psoriatic arthritis, the results of this trial do not support abatacept as a choice for treating psoriatic arthritis and psoriasis skin lesions concurrently,” concluded Harris et al.
In an accompanying editorial, Kamran Ghoreschi, MD, of Charité–Universitätsmedizin Berlin, and corporate member of Freie Universität Berlin and Humboldt Universität zu Berlin, Germany, explained that this translational study from Harris et al illuminates several important conclusions.
First, abatacept is not effective in preventing psoriasis relapse after ustekinumab withdrawal in patients with PASI 75 response. Next, “From an immunological perspective, the manipulation of checkpoint pathways is less beneficial for the inflammatory signature than the manipulation of cytokine pathways, which may also explain why the manipulation of checkpoint pathways may be less successful in disease relapse,” noted Ghoreschi.
In addition, IL-12/IL-23p40 is “probably not the ideal target,” he added.
Nevertheless, these results are important in the continued and expanded understanding of psoriasis and the immunologic factors that play a role in its etiology, progression, and relapse.
“Translational studies such as that by Harris and colleagues help us identify molecular signatures to optimize treatment regimens and, with hope, to prevent psoriasis relapses with personalized strategies,” concluded Ghoreschi.
Study limitations include the possibility that the abatacept dose may have been too low or administered too late after ustekinumab to prevent relapse and a limited number and frequency of paired skin and serum samples. Another limitation is that disease duration was neither an inclusion nor an exclusion criterion, added Ghoreschi.
-
PAUSE researchers found that treatment with abatacept after ustekinumab withdrawal did not prevent psoriasis relapse in patients with moderate-to-severe plaque psoriasis.
-
Abatacept is approved for psoriatic arthritis, but these results do not support its use for treating psoriatic arthritis and psoriasis skin lesions concurrently.
Liz Meszaros, Deputy Managing Editor, BreakingMED™
This trial was conducted by the Immune Tolerance Network and supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH). Abatacept and abatacept placebo were provided by Bristol Myers Squibb. Multiplex serum cytokine analysis was conducted by Meso Scale Diagnostics LLC and supported by the NIAID/NIH. IL-19 serum assay was conducted and supported by Eli Lilly and Co.
Harris reported no disclosures.
Ghoreschi reported receiving personal fees for participation in advisory boards or as speaker as well as grants as an investigator for AbbVie, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, and UCB.
Cat ID: 10
Topic ID: 75,10,730,10,192,919,925