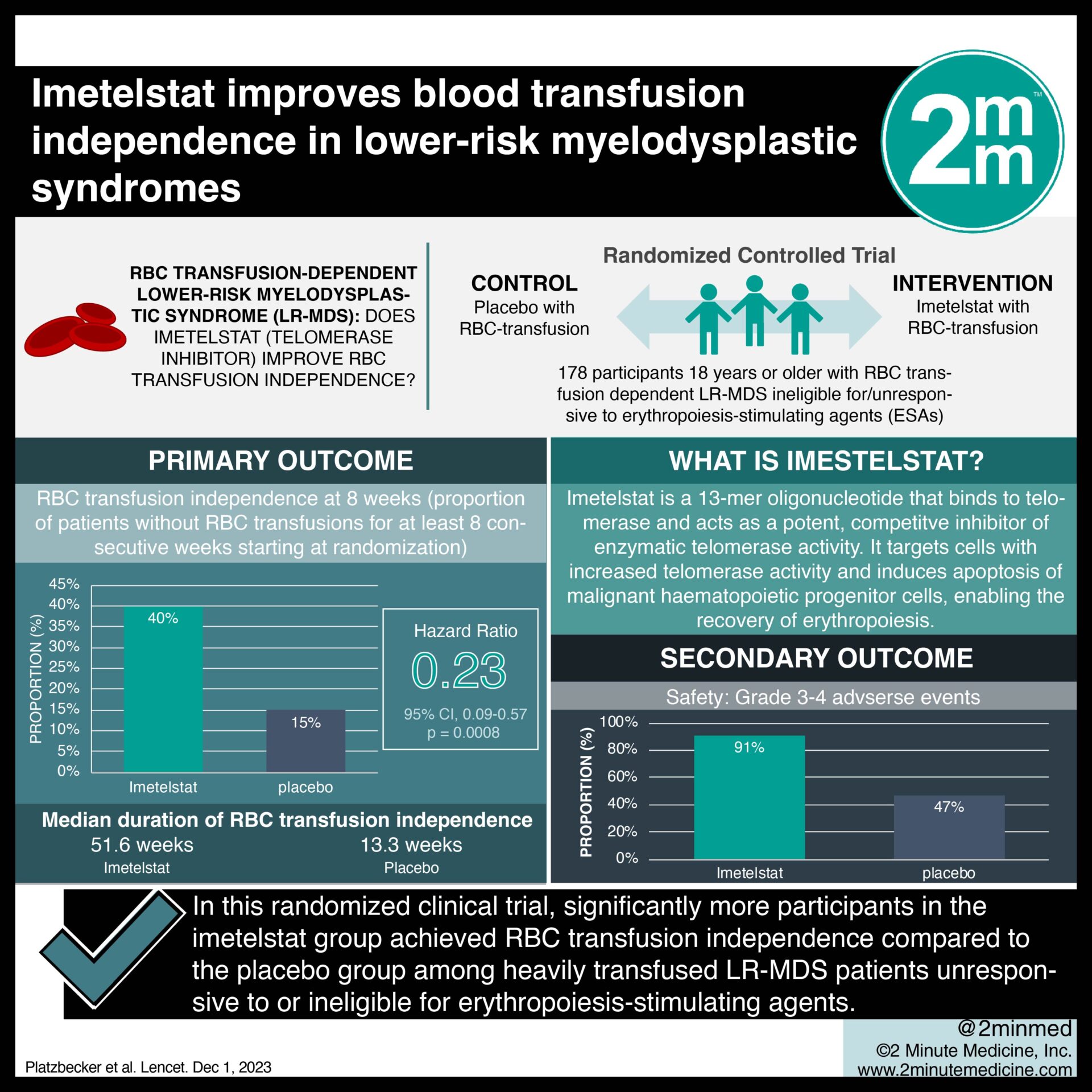
1. Significantly more patients in the imetelstat group achieved RBC transfusion independence at 8 weeks after treatment compared to placebo.
2. Grade 3-4 treatment-related adverse events were greater in the imetelstat group.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Patients with red blood cell transfusion-dependent lower-risk myelodysplastic syndromes (LR-MDS) face unmet medical needs when unresponsive to erythropoiesis-stimulating agents (ESAs). Imetelstat, a telomerase inhibitor, showed promise in a phase 2 trial; however, limited evidence exists surrounding its therapeutic efficacy. This randomized controlled trial aimed to compare imetelstat with placebo in ESA-relapsed, ESA-refractory, and ESA-ineligible LR-MDS patients. The primary outcome was RBC transfusion independence (RBC-TI) at 8 weeks, while key secondary outcomes were the rate of grade 3-4 treatment-emergent adverse events. According to study results, imetelstat demonstrated a higher 8-week RBC-TI rate compared to placebo but was associated with more severe adverse advents. Although this study was well done, it was limited by a high discontinuation rate in both groups.