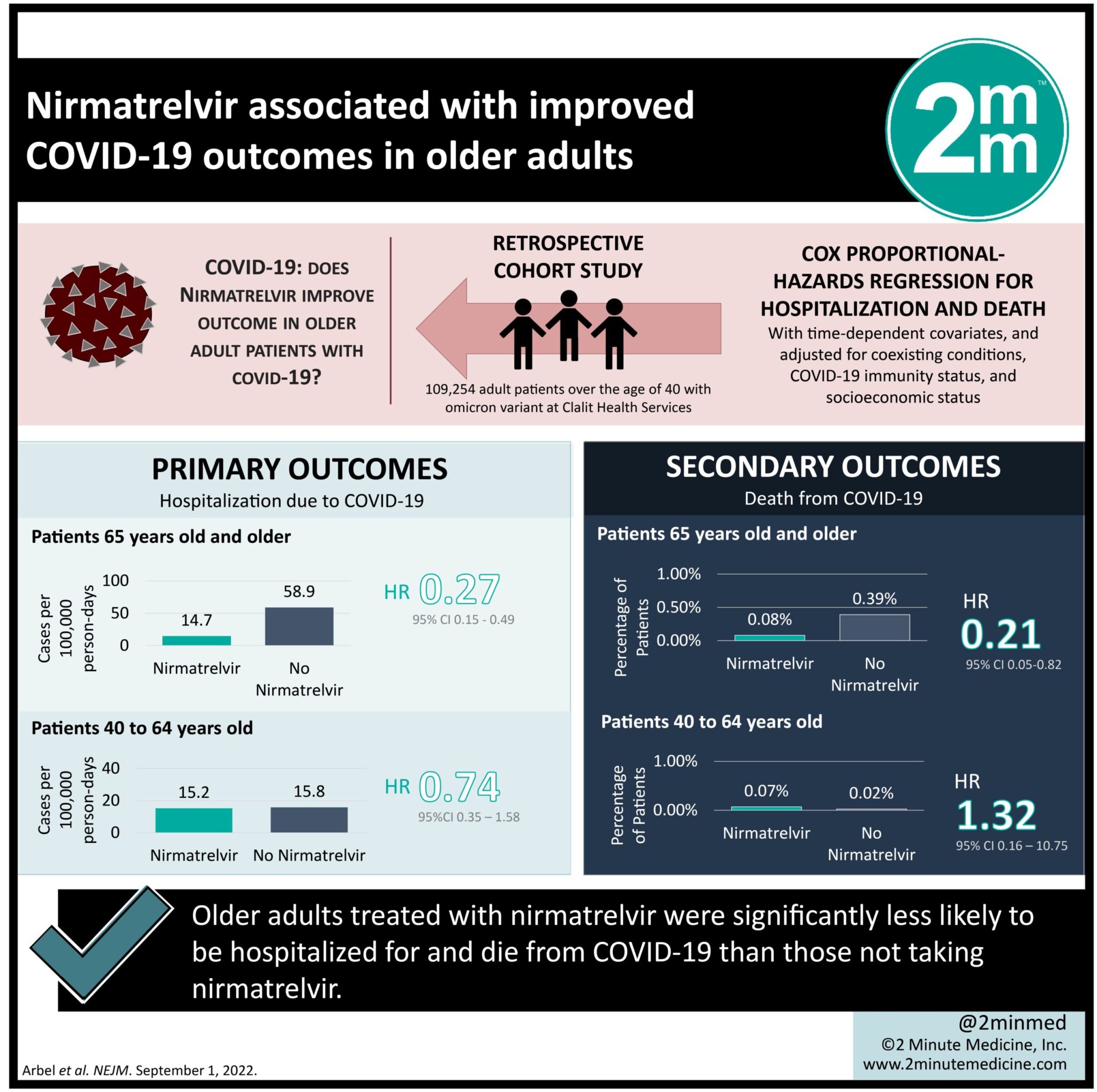
1. Older adults treated with nirmatrelvir were significantly less likely to be hospitalized for coronavirus disease 2019 (COVID-19) than those not taking nirmatrelvir.
2. Those in the nirmatrelvir group were also significantly less likely to die from COVID-19 infection.
Evidence Rating Level: 1 (Excellent)
Study Rundown: The omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a worldwide resurgence of COVID-19. Nirmatrelvir was approved by the Food and Drug Administration (FDA) for the treatment of patients with mild-to-moderate COVID-19 when the delta variant was the predominant strain. This study examined its effectiveness in patients with the omicron variant above the age of 40. Adults above the age of 65 treated with nirmatrelvir were significantly less likely to be hospitalized or die from COVID-19 than those not on treatment. Older adults who also had previous immunity were significantly less likely to be hospitalized for COVID-19 than those without previous immunity. Among patients in the younger and older adult age groups, previous hospitalization and immunity were the most strongly associated with hospitalization due to COVID-19. This study suggests that treatment with nirmatrelvir for older adults with a high risk for progression to severe COVID-19 can reduce hospitalization and death. A limitation of this study is that only a minority of patients identified as high risk of progressing to significant disease were provided treatment with nirmatrelvir. This may result in a selection bias in the observed population and may lead to an underestimated treatment effect in this study.
Click to read the study in NEJM
Relevant Reading: Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19
In-Depth [retrospective cohort]: The present observational, retrospective cohort study evaluated the efficacy of nirmatrelvir for treating COVID-19 infection in adults over the age of 40. A total of 109,254 of patients were eligible for this study. The mean study age was 60 years. In the study population, 78% of patients had previous immunity due to vaccination, previous infection, or both. Risk factors that led to hospitalization in the older adult population included recent hospitalization, obesity, diabetes, chronic hepatic disease, neurologic disease, chronic heart failure, chronic obstructive pulmonary disease, history of stroke, and chronic kidney failure. The adjusted hazard ratio for hospitalization in older adults was 0.27 (95% Confidence Interval [CI], 0.15 to 0.49). For adults aged 40 to 64 years old, the adjusted hazard ratio for hospitalization was 0.74 (95% CI, 0.35 to 1.58). Older adults who received nirmatrelvir and have previous immunity had an adjusted hazard ratio for hospitalization of 0.15 (95% CI, 0.04 to 0.60). Among patients 65 and older who were treated with nirmatrelvir compared to untreated, the adjusted hazard ratio for death was 0.21 (95% CI, 9.05 to 0.82). In patients aged 40 to 64 years who were treated with nirmatrelvir compared to untreated, the adjusted hazard ratio for death was 1.32 (95% CI, 0.16 to 10.75). The greatest risk factor for hospitalization for both the younger (aHR 5.79, 95% CI, 4.58 to 7.32) and older (aHR 5.82, 95% CI, 4.99 to 6.78) age group was no previous immunity. For adults over the age of 65 during the omicron stage of COVID-19, treatment with nirmatrelvir was associated with significantly lower severe outcomes than those who did not receive nirmatrelvir treatment.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.