2. The bepirovirsen group had significantly increased adverse events compared to the placebo group.
Evidence Rating Level: 1 (Excellent)
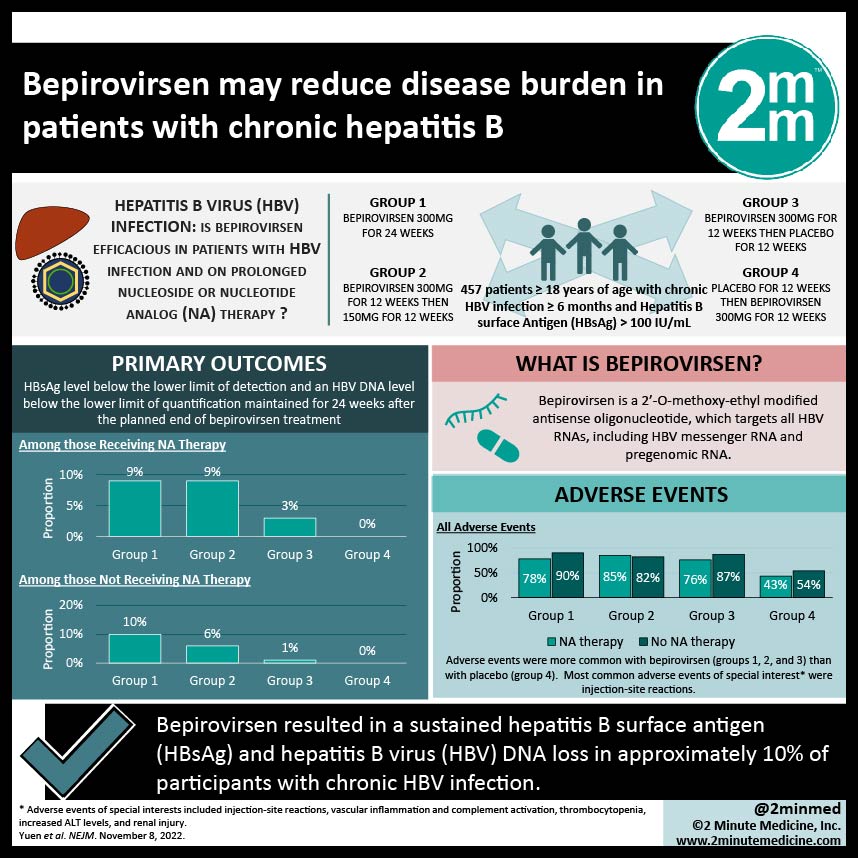
Study Rundown: Chronic HBV infection causes approximately 820,000 deaths yearly, with an estimated 1.5 million new infections annually. While therapy aims to achieve a functional cure, with HBsAg loss or sustained undetectable HBV DNA after cessation of therapy, this is uncommon in patients receiving prolonged treatment. In patients receiving prolonged nucleoside or nucleotide analog (NA) therapy (the first-line treatment for HBV), fewer than 5% of patients have HBsAg loss after 12 months of treatment. Bepirovirsen is a 2’-O-methoxy-ethyl modified antisense oligonucleotide, which targets all HBV RNAs, including HBV messenger RNA and pregenomic RNA. However, there is a gap in knowledge as to understanding the efficacy and safety of 12- and 24-week bepirovirsen treatment in participants with chronic HBV infection either receiving stable NA therapy or not receiving NA therapy. Overall, this study found that treatment with bepirovirsen resulted in HBsAg and HBV DNA loss for 24 weeks after the end of treatment. This study was limited by having short follow-up and a small participant pool. Nevertheless, these study’s findings are significant, as they demonstrate that bepirovirsen may be efficacious in treating chronic HBV in inducing a sustained loss of HBsAg and HBV DNA.
Click to read the study in NEJM
Relevant Reading: A Modern Therapy for an Ancient Disease
In-Depth [randomized controlled trial]: This phase two randomized, parallel cohort trial was conducted at 123 sites in 22 countries. Participants 18 years of age or older with documented chronic HBV infection for at least six months and an HBsAg level of more than 100 IU per milliliter were eligible for the study. The primary outcome measured was an HBsAg level below the lower limit of detection and an HBV DNA level below the lower limit of quantification maintained for 24 weeks after the planned end of bepirovirsen treatment. There were four treatment groups: bepirovirsen 300mg for
24 weeks (group 1), bepirovirsen 300mg for 12 weeks then 150mg for 12 weeks (group 2), bepirovirsen 300mg for 12 weeks then placebo for 12 weeks (group 3), and placebo for 12 weeks then bepirovirsen 300mg for 12 weeks (group 4). Based on the primary analysis, among those receiving NA therapy, a primary-outcome event occurred in six participants in group 1 (9%; 95% credible interval, 0 to 31), six in group 2 (9%; 95% credible interval, 0 to 43), two in group 3 (3%; 95% credible interval, 0 to 16), and zero in group 4 (0%; post hoc credible interval, 0 to 8). Among participants not receiving NA therapy, a primary-outcome event occurred in seven (10%; 95% credible interval, 0 to 38), four (6%; 95% credible interval, 0 to 25), one (1%; post hoc credible interval, 0 to 6), and zero participants (0%; post hoc credible interval, 0 to 8), respectively. Adverse events were more common with bepirovirsen (groups 1, 2, and 3) than with placebo (group 4). Overall, this study demonstrates that bepirovirsen at a dose of 300mg per week for 24 weeks resulted in sustained HBsAg and HBV DNA loss in 9-10% of participants with chronic HBV infection.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.


